
 |
 |

|
Here we talk about the Boltzmann constant. Vague
physical essence of the constant allows to explain and describe various
effects in different media when it’s necessary.
It’s connected with thermal radiation in gas medium, in
liquids it’s the essence of the Brownian motion, and it
justifies current noise in metals and thermal currents in
semiconductors.
This fact alone indicates poor understanding of the constant origin and its wrong use in theories. What makes the monkey from Krylov’s fable use the glasses so wrongly? Obviously, it is the attractiveness of the glasses and absence of knowledge how to use them. Chapter 1. Missing Probability. . My boxing idol is Kostya Tszyu. In one of the TV commercials he explains why a straight blow is more effective than a hook. He is sure (along with other boxers) that the point is in the length of the fist way. The straight way is shorter, which means that a boxer delivers a blow faster. However they forget that different blows are made by different muscles. These muscles differ in force by 10 times so such an explanation is false. The way length of the mentioned blows differs just by 30-40% and for some boxers it’s more convenient to use a hook. So is Kostya wrong? Not at all. He just explains it incorrectly. A boxer forms reaction for better defense which allows them to dodge an opponent’s blow in time. Reaction requires detection of a fist move. Stereovision of our eyesight leads to detection of a fist move by change of its angle size. In case of a straight blow the move is detected when the fist is already in the middle of the way while a hook is seen at once. The path of indirect motion becomes apparent by angular deflection. It’s very good that the conclusion was right despite of wrong understanding of the cause. Mere coincidence. But here we have just two options, but what if there were 10 or 100? What is the value of hit probability? Right, almost zero. Chapter 2. Bogs & Morasses. . Modern scientific articles are usually decorated with ‘the Clapeyron or Schrödinger equations’, ‘the Gibbs laws’, ‘the Pauli exclusion principles’, etc which while being read impose on the reader unphysical nature, a gap between the topic and nature, and formal knowledge. These rules and laws allows some kind of voluntarism. If each of these rules has a feature of ‘missing’ to some extent, science as a whole moves in the wrong direction if not backwards. Recently I have read that the International Committee of Weights is planning to abandon the existing definition of the Kelvin degree and substitute it for the function of the Boltzmann constant [1]. This decision was caused be the experiments of French scientists that allow to measure the Boltzmann constant accurate within 10 -6 using acoustic speed in argon. No problem, it’s possible to use any stable mark as a reference. But first it’s necessary to prove that it is stable and there is 100% correlation between the mark and the test parameter. There are a lot of examples in the history of science showing that guess-work is unacceptable. Let’s recall positive current ‘from plus to minus’. It was a wrong variant that was chosen from the two possible ones. It doesn’t seem to be serious; according to the wrong current direction several rules were accepted: the right-hand screw, the clockwise rule, and the right- and left-hand rule. And when they found out the real current direction, it was too late to change all the rules. So we live with all that staff – there are only exceptions, effects and rules that do not correspond to the real state of affairs. I also would like to remind about the Rydberg ‘constant’ R = 109737,316 cm-1. It’s unknown how they could find a formula for it (CGS) But it is known that the experimentally measured wavelengths didn’t match the calculations to some extent. The corrections for different atoms were introduced: " Hydrogen: RH = 109677,593 cm-1; " Deuterium: RD = 109707,417 cm-1; " Helium: RHe = 109722,267 cm-1. The discrepancy was justified (supposedly) by the so called normalized value of electron mass. The normalized value didn’t match either, but they didn’t manage to do more. Later it was found out that mass couldn’t be normalized and the Rydberg constant wasn’t a radiation constant at all, but hidden energy of atom ‘ionization’. And again it was too late to change anything. The example of the de Broglie law is hardly better. Sucked down in the bog of mistakes. There is no need to worsen the matter with Kelvin. Chapter 3. Real researches. . Real doesn’t mean absolutely true. Real researches are not supposed to use postulates and hypotheses in tools. It means that it is possible to act using only natural laws and rules obtained by the same way. Our researches are to be considered a search for the path leading to the goal. Let’s go back to the Boltzmann constant. Who and when identified radiation, noises, the Brownian motion kinetics and thermal currents? Nobody did it publicly, but everyone could use the Boltzmann constant as one wished without fearing any prohibitions exactly because of absence of nature of its origin. It is the gap that we will try to bridge. 3.1. Gram mole volume Interaction between charged particles is known according to Coulomb’s law, its more correct representation through energy is given in [2]. The law is formulated for free electrons, but atoms have energy as well. It is the energy that is responsible for interaction between atoms and molecules. This interaction is based on the so called nuclear (protonic to be exact) nature that creates attractive forces. Due to that molecules are created in gases and crystal lattices - in metals. There is no atom ionization, and there is no need for it. Ionization is not emission of electrons, but change of atom size. (See "New Understanding of Franck-Hertz experiment"). The nature of charged particles interaction can be demonstrated using an example of an electron, its energy field formula is Pay attention to two facts: " Nature ‘measures’ distances not in human units of length, but in relative ones, compared to its size which are different for different energy values; " Numerator is a constant value, it means that if particle energy changes, field energy doesn’t change on the same radius. It allows us to dispel all the doubts about the stability of gas volume V 0 (22,4 l) of a substance gram mole. Let’s calculate the distance between gas molecules where А- the Avogadro constant. The determined value is 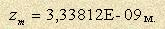 3-2. Air Composition. Air consists of the three main components: Oxygen - 20,95 % (by volume); Nitrogen - 78,09 %; Argon - 0,933 %. Naturally, these components contribute different amount of energy to the space. 3-3. Generalized field of air energy. Let’s cite the reference data for the participating atoms (Table 1).
Atom binding energy is determined as half energy of molecular dissociation. Energy of a single atom is taken as energy of ‘first ionization’. Atom energy in a molecule is atom energy minus binding energy. The generalized value of ambient energy E0 is a sum of energy contribution of its components multiplied by volume fraction of the component The absolute value of the difference between molecular residual energy and Е 0 energy is an energy source for work of any kind (Table 2).
The sum of the values of difference energy is also responsible for intermolecular binding. Intermolecular binding energy is calculated with the help of the formula similar to (2) In the general case the number of such bonds around a molecule is 4π. So residual energy of an average atom On per a Kelvin temperature scale degree basis it is 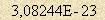 J. This energy is to be divided in usage directions.
J. This energy is to be divided in usage directions. 3-4. Physics of atoms and molecules. Those who are familiar with the electron model [3], know that field energy of an electron consists partly of orbital energy of magnetic forces and partly of high-frequency meridional magnetic forces. Orbital forces are responsible for formation of vortex magnetic field while meridional ones are responsible for linear motion of an electron in electric field. Vortex magnetic field provides electromagnetic radiation as well. An atom has in essence the same characteristics as an electron, but here we talk about molecules that brings some nuances to the matter. The first one is relative atom orientation in a molecule. It is possible only as antiparallel one, i.e. when equators roll around each other. The second one is molecule massiveness that doesn’t allow it to make vibration reducing for external forces. 3-5. Components of energy consumption. Concerning the arguments discussed above, half of residual atom energy should be attributed to radiation energy 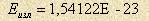 J. The other half must be kinetic energy. But due to the nuances
mentioned above this energy is almost impossible to realize. Acting
magnetic forces meet strong molecular resistance, so the
lion’s share of energy turns into heat. If this energy turned
into motion, molecules would have the speed of 20 m/s. These speeds are
obviously not Brownian, so we attribute negligibly small amount to
Brownian motion.
J. The other half must be kinetic energy. But due to the nuances
mentioned above this energy is almost impossible to realize. Acting
magnetic forces meet strong molecular resistance, so the
lion’s share of energy turns into heat. If this energy turned
into motion, molecules would have the speed of 20 m/s. These speeds are
obviously not Brownian, so we attribute negligibly small amount to
Brownian motion.Thus, radiation energy of gas molecules is indeed characterized by a value approximately equal to the Boltzmann constant. The result that we obtained differs from this constant by 11%, but we can’t claim to have the highly accurate analysis. Firstly, we used the experimental values of atom radii and their energies which are rather different in the references. Secondly, we have hardly considered all the factors. Still we have found out the key point: the Boltzmann constant isn’t a constant, because it is connected with the percentage composition of its components and the type of molecular and intermolecular bonds. As for the French scientists’ achievement, the high accuracy of the argon measurements is caused by the fact that it is a monoatomic molecule which isn’t characterized by the nuances mentioned above. Conclusions: 1. It is unacceptable to use the Boltzmann constant for standardizing a temperature scale as it is not a physical constant.Использовать постоянную Больцмана для эталонирования температурной шкалы недопустимо, т.к. она не является физической константой. 2. It is unacceptable to use the Boltzmann constant for calculating kinetic energy of ambient molecules. 3. It is unacceptable to use the Boltzmann constant for charactering currents in semiconductors. BIBLIOGRAPHY 1. Novii metod izmereniya konstanti Boltzmana pozvolit izmenit opredelenie gradusa Kelvina [New Method of Boltzmann Constant Measuring to allow Kelvin Degree Change]. http://forextimes.ru/news/hnews136125.htm 2. Rudnev A. D. Shag k structure prostranstva [Step towards Space Structure]. http://www.sciteclibrary.ru/rus/catalog/pages/8182.html 3. Rudnev A. D. Novaya kontseptsiya fisiki [New Physics Concept]. http://www.sciteclibrary.ru/rus/catalog/pages/6910.html |
