
 |
 |

|
Atomic radiation spectra
are a powerful
tool of remote sensing of substance composition. Every substance has
its own setting of radiation frequencies called spectrum lines. They
are steadily stable, so they durably transmit valuable information
about composition of substances, alloys, gases and even planets in the
space. Nobody can call the functionality of the origin of this
radiation in question (i.e. everybody must deny the probabilistic
principle of atomic structure). Still nobody can explain either origin
of radiation or principles of simultaneous transmission of spectrum
radiation and absorption. The situation is like this because nobody
knows atomic structure.
As soon as we found out [1] that electrons follow the law, and then we discovered that radiuses of outer atomic electrons can’t be different one from the other, we are forced to admit that an atom is an electron sphere, common for all electrons. We also found out that meridian fluctuations of electron energymass have the equal phase displacement in the period, that is why Gaussian law becomes true and the functionality of the processes in the atom recovers. Magnetic forces of medium resistance become stochastic for an atom because a number of meridian fluctuations of energymass in the period can’t be a whole number (fine structure constant is an irrational number). As a result we have jitter of atomic electron sphere – phonons. It must be mentioned that it’s not an atom that jitters, but an electron sphere only. A nucleus is almost immovable. Now let’s prove that orbital revolution of an electric charge causes a radiation wave contrary to Bohr’s statements. Though Bohr spoke about electron revolution in an orbit, but this idea was caused by the fact that an electron was considered a structureless charge, that’s why electron energymass is essentially a “Bohr electron”. In this case atom radiations, as a result of circular interaction of electron energymass with the medium, must be absorbed by the same medium. For a fixed observer these radiations are periodic magnetic force in the space. Then free electrons of the medium are to create undular radiation themselves in the same way with the period Let’s check the correctness of our conclusions. It can be done by calculating not frequency, but wavelength of a free electron What is it? It’s de Broglie wavelength, registered long time ago, but having some esoteric explanation saying that it is connected with mass of bodies which radiate as well according to this hypothesis. And it’s just a vibration of the space, response of free electrons interaction. It’s already known that orbital revolution of electron energymass at the speed 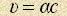 determined
the period
value determined
the period
value It means that electrons radiate by orbital revolution of energymass as any charge movement responds with reaction of magnetic forces and centripetal ones as well. Again we say “ha” to classical physics opponents and again note that real physics doesn’t need any hypotheses and postulates. All the facts are within physics rules and stop being effects. Now let’s explain the existence of the Planck constant. Electrons and atoms interact with the environment by means of magnetic forces and intensity of a magnetic field is characterized by electric field. It results in the fact that time correlation of electric and magnetic fields of common source is possible only in discrete instants in time multiple of the orbital revolution period of enrgymasses. The reason is that magnetic parameters of the field don’t have process “power”, they are expressed only by energy, integral work of magnetic forces during the process period. In this case electric or magnetic particle energy is to be multiplied by the period to find out the correlation with integral magnetic energy. The most convenient period of periodic processes in a particle (the smallest common measure) is an orbital revolution period of energymasses which is expressed through the radius and constant orbital speed 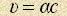 Therefore the common measure of particle energy is a product of the energy and this period. It’s possible to calculate the product if a particle of known radius and energy is chosen. By substituting these values for an electron we get Now let’s prove that the so called Rydberg constant is nothing other than an implicit form of notation of hydrogen atom energy. It follows from the fomula Er=Const that the enrgy of an hydrogen atom is to be equal 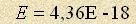 J (27,2 electron volt).
It’s necessary to explain that total electron energy
isn’t mc2, as it generally assumed, but thrice as much. Due
to energy parameter quantization an atom didn’t reach the
total double value with a corresponding radius that is twice as less
than a Bohr one. It’s the very reason why Bohr tried to fit
the value of hydrogen atom energy. (refer to "Bohr Rutherford Myth").
The natural value of hydrogen atom energy is J (27,2 electron volt).
It’s necessary to explain that total electron energy
isn’t mc2, as it generally assumed, but thrice as much. Due
to energy parameter quantization an atom didn’t reach the
total double value with a corresponding radius that is twice as less
than a Bohr one. It’s the very reason why Bohr tried to fit
the value of hydrogen atom energy. (refer to "Bohr Rutherford Myth").
The natural value of hydrogen atom energy is 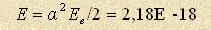 J. Let’s take
the primary radiation wavelength J. Let’s take
the primary radiation wavelength 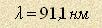 of a
hydrogen atom and
make sure it corresponds precisely with the period of orbital atom
radiation of a
hydrogen atom and
make sure it corresponds precisely with the period of orbital atom
radiation 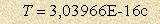 . .The unsatisfactory condition “energy-radius” of a hydrogen atom leads to the situation when an electron is able both to release some of its energy to fit the Bohr radius, and to absorb additional energy to fit the double value of the Bohr radius. That is all there is to it. The problem concerning “ionization” of a hydrogen atom, when there is no and can be any electron transmission, is solved. However, a union of two hydrogen atoms results exactly in mutual loss of atom “connection” energy that is why formation of hydrogen molecules becomes the “desired” process. Still there is a paradox: normalization of energy-radius correlation doesn’t have a solution for the integral radius change according to the laws of motion. As a result, a search of atom radius begins by fractions and right here we have the Ritz algorithm - 1/n. Let’s make conclusions without going into much details: This process is periodical and infinite as there is no final solution. Atoms release energy during the first half of the process and absorb it during the second one. Hence there are absorption and radiation lines in the atomic spectrum. Constant atom radius change under condition of interatomic bond makes the bond radius in a molecule smaller. As a result, there is the so called ”tunnel effect” of atomic nuclei. You can see the diagram of atomic energy state in a hydrogen molecule in picture 1. The cycle period consists of 22 stroke. The first 11 strokes are energy absorption from the environment. Pic. 1. Diagram of atom
parameters energy adaptation in a hydrogen molecule. There are a lot of details which are impossible to cover here, let’s leave them for the future. One of them is the magic of 21 cm line disappearing in a molecule. Another is connected with wavelength correction calculated according to the Ritz algorithm. Bibliography : 1. Rudnev A. D. "Shag k structure prostranstva (V osnove strukturi prostranstva – elektroni)" [A Step towards Space Structure (Electrons are the Basis of Space Structure)]. http://www.sciteclibrary.ru/rus/catalog/pages/8253.html |
